+982188957681
+982188957685
+989126754593
Wastewater Treatment

Magnesium oxide and magnesium hydroxide can both be used in various ways for the treatment of wastewater
In order to repel the unpleasant odor from waste water its main sources H2S and ammonia must be eliminated. This can be achieved by the introduction of MgO in the system with a buffer solution of 9-9,5 pH prohibiting in this way the formation of H2S.
Corrosion of the utilities networks or Struvite tanks is also attributed to the formation of sulfuric acid (H2SO4) alkaline magnesium reagents can help in their protection either with continuous addition magnesium oxide/hydroxide in parts of the network for pH adjustment or through coating the free surface of the pipes with magnesium hydroxide slurry.
The removal of phosphorus is of major interest lately since phosphates interfere with the phenomenon of eutrophic and lead to the death of aquatic life. Additionally, due to the decrease of natural phosphate deposits the recovery and recycling of phosphorous has become desirable.
The byproduct of the phosphorus removal is called struvite and could be used as a fertilizer (Mg, N, P source). Magnesium ions provoke the increase of the chlorophyll production since they occupy the central position in its molecule, while N and P are important nutrients for the plants. In that way the treatments byproduct can be sold as fertilizer making the whole treatment process even more profitable.
It should be noted that the use of MgO / Mg(OH)2 achieves the best possible recovery yield of the phosphates in comparison to Ca(OH)2/CaO due to more effective pH control.
Blogs

12/25/2018
Magnesium sulfate or magnesium sulphate
Magnesium sulfate (or magnesium sulphate) is an inorganic salt (chemical compound) containing magnesium, sulfur and oxygen, with the formula MgSO4. It is often encountered as the heptahydrate sulfate mineral epsomite (MgSO4·7H2O), commonly called Epsom salt, taking its name from a bitter saline spring in Epsom in Surrey, England, where the salt was produced from the springs that arise where the porous chalk of the North Downs meets non-porous London clay. The monohydrate, MgSO4·H2O is found as the mineral kieserite. The overall global annual usage in the mid-1970s of the monohydrate more ...
12/25/2018
Our Product Heavy Element Content
Heavy elements as toxic elements for animals and plants in magnesium carbonate and oxide have to be very low in content and our products overcome the standard factors! more ...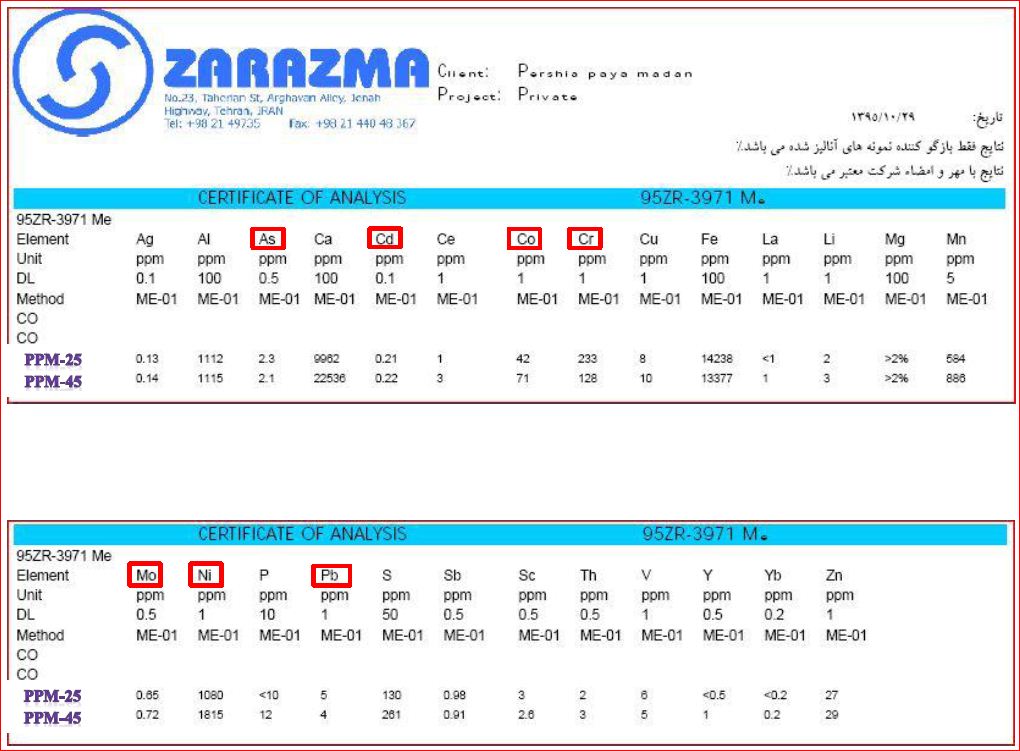
12/25/2018
Table analysis of products for livestock and poultry
According to specific conditions for livestock and poultry feed market, different magnetite products are classified for different applications which are presented on attached table.Due to low levels of heavy metals in our products (see Table attached), we are proud of supplying a wide range of Livestock and Poultry feed market and with supplementation of a high standard, homogeneity and stable conditions. more ...

5/24/2018
Magnesite
Magnesite is a mineral with the chemical formula MgCO3 (magnesium carbonate). Mixed crystals of iron(II) carbonate and magnesite (mixed crystals known as ankerite) possess a layered structure: monolayers of carbonate groups alternate with magnesium monolayers as well as iron(II) carbonate monolayersManganese, cobalt and nickel may also occur in small amounts.Occurrence
Magnesite occurs as veins in and an alteration product of ultramafic rocks, serpentinite and other magnesium rich rock types in both contact and regional metamorphic terrains. These magnesites often are cryp more ...

5/24/2018
Magnesite Applications
Magnesite occurs as veins in and an alteration product of ultramafic rocks, serpentinite and other magnesium rich rock types in both contact and regional metamorphic terrains. These magnesites often are cryptocrystalline and contain silica in the form of opal or chert.Magnesite is also present within the regolith above ultramafic rocks as a secondary carbonate within soil and subsoil, where it is deposited as a consequence of dissolution of magnesium-bearing minerals by carbon dioxide within groundwaters.
Similar to the production of lime, magnesite can be burn more ...

5/24/2018
Heavy element
Heavy element contentConsidering the specific requirements of defining the products for the livestock and poultry market after consulting the technical and commerce section of the annex for this market was designed
Given the low amounts of heavy metals in products Appendix Table we are proud to provide the market for animal feed and complementary products with a high standard of production uniformity
Our two best-selling products in Animal Feeding market include magnesium carbonate PPM-25 and magnesium oxide CCM PPM-45 whose analysis of heavy elements is more ...

5/24/2018
Magnetite Deposits
Magnetite is relatively frequent as a mineral, there are, however, only two types of economically important deposits:The worlds largest reserves are found in deposits of the Veirsch type. These are strata-bound lensoid and nearly monomineralic bodies consisting of coarsely crystalline ,,spar,, magnesite hosted by marine platform sediments. Conspicuous are sedimentary and diagenetic features of the magnesite bearing suites, which indicate formation within a subtidal, evaporitic environment closely associated with sabkhas, mudbars or other inter- to supratidal highs. The coarse c more ...

5/24/2018
Magnesium oxide
Magnesium oxide, or magnesia, is a white hygroscopic solid mineral that occurs naturally as periclase and is a source of magnesium.Formula: MgO
Molar mass: 40.3044 g/mol
IUPAC ID: Magnesium oxide
Melting point: 2,852 °C
Density: 3.58 g/cm³
Boiling point: 3,600 °C
Magnesia or magnesium oxide is an alkaline earth metal oxide. The majority of magnesium oxide produced today is obtained from the calcination of naturally occurring minerals. Magnesite, MgCO3, being the most common.
Both MgCO3 and Mg(OH)2 are converted to MgO by calcination. T more ...
4/3/2018
Links for Each Magnesia Application
Agriculturalhttp://blog.magspecialties.com/category/applications/agricultural/
Electric Grade Steel
http://blog.magspecialties.com/category/applications/electric-grade-steel/
Electric Utilities
http://blog.magspecialties.com/category/applications/electric-utilities/
Flame Retardant / Smoke Suppressant
http://blog.magspecialties.com/category/applications/flame-retardant-s more ...

4/3/2018
Wastewater Treatment
Magnesium oxide and magnesium hydroxide can both be used in various ways for the treatment of wastewaterIn order to repel the unpleasant odor from waste water its main sources H2S and ammonia must be eliminated. This can be achieved by the introduction of MgO in the system with a buffer solution of 9-9,5 pH prohibiting in this way the formation of H2S.
Corrosion of the utilities networks or Struvite tanks is also attributed to the formation of sulfuric acid (H2SO4) alkaline magnesium reagents can help in their protection either with continuous addition magnes more ...

4/3/2018


